Coagpia Fbg
We created this page for medical staff.
- *Read carefully the package insert before use.
Performance Data
Within-run reproducibility
| Sample 1 | Sample 2 | Sample 3 | |
|---|---|---|---|
| 1 | 253 | 124 | 84 |
| 2 | 249 | 116 | 81 |
| 3 | 255 | 120 | 82 |
| 4 | 249 | 115 | 82 |
| 5 | 259 | 115 | 85 |
| 6 | 255 | 115 | 85 |
| 7 | 259 | 113 | 88 |
| 8 | 261 | 116 | 79 |
| 9 | 247 | 117 | 87 |
| 10 | 255 | 123 | 85 |
| Mean | 254 | 117 | 84 |
| S.D. | 4.7 | 3.7 | 2.8 |
| C.V. (%) | 1.9 | 3.1 | 3.3 |
| Max. | 261 | 124 | 88 |
| Min. | 247 | 113 | 79 |
| Range | 14 | 11 | 9 |
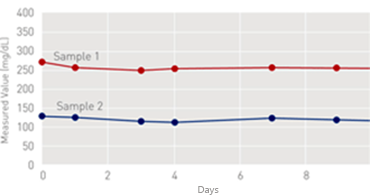
On-Board Stability
[on Coapresta 2000]
Interference
| Concentration | Measured Value(mg/dL) | ||
|---|---|---|---|
| Base Plasma | Spiked Plasma | ||
| Unconjugated Bilirubin | 25 mg/dL | 270 | 281 |
| Conjugated Bilirubin | 25 mg/dL | 270 | 279 |
| Hemoglobin | 500 mg/dL | 328 | 330 |
| Chyle | 3000 FTU | 270 | 280 |
| Heparin Sodium | 8 U/mL | 270 | 259 |
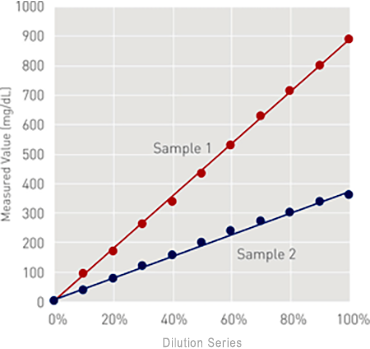
Linearity
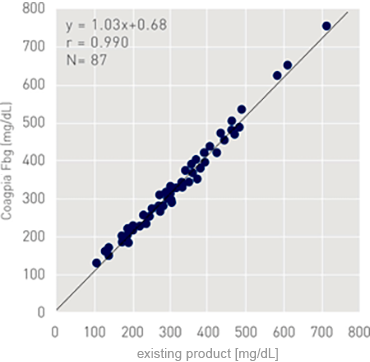
Correlation
Traceability - Accuracy Verification of Calibrator N for Coagpia
Measured values of Calibrator N for Coagpia were verified using WHO reference material as a calibrator. The closer the measured value is to the calibrator's assigned value (i.e. recovery is closer to 100%), the more closely the sample resembles the reference. Calibrator N for Coagpia was shown to precisely resemble the WHO reference material.
- Reagent: Coagpia Fbg
- Calibrator: WHO 2nd international standard for plasma Fibrinogen NIBSC CODE 98/612 Assigned value 295 mg/dL
- Analyzer: Coapresta 2000
| Sample | Measured value with Coagpia Fbg(mg/dL) | Mean(mg/dL) | Recovery (%) |
|---|---|---|---|
| Calibrator N for Coagpia Lot A Assigned value 295mg/dL |
305 | 299.0 | 101.4 |
| 287 | |||
| 305 | |||
| Calibrator N for Coagpia Lot B Assigned value 296mg/dL |
298 | 305.3 | 103.5 |
| 309 | |||
| 309 |
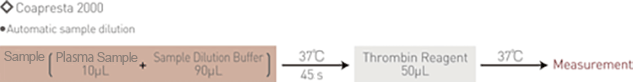
Assay Procedure
- Automatic sample dilution
"Coagpia" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
"Coapresta" and all related logos are trademarks or registered trademarks of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.