Delivering safe and reliable medicines to patients as quickly as possible
As medical needs are diversified, and different treatments are required,
the roles of medicines have become very important and diverse.
We have responsibilities to deliver safe and reliable medicines to patients immediately.

As medical needs have diversified with the evolution of pharmaceutical sciences, we are expected to respond to various types of disease. Under such circumstance, the roles and expectations of medicines which protect lives are growing, there is a constant need for absolute safety, security, and speed to deliver them as quickly as possible. Society also demands that we develop medicines with new efficacy for diseases that cannot be treated before with conventional medicines.
As a contract manufacturing organization in this pharmaceutical field, we support the foundation to produce active pharmaceutical ingredients (APIs), which form the basis of drugs, and investigational drugs for clinical trials to support the development of new medicines.
Flow of Contract Manufacturing Organizations and Sekisui Medical's domain
What is a Contract Manufacturing Organization?
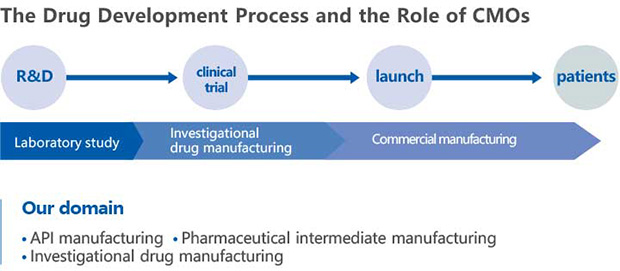
Contract manufacturing organizations (CMOs) are companies that contract with pharmaceutical companies to manufacture drugs (including investigational drugs and over-the-counter drugs). Thus, pharmaceutical companies, which incur huge costs to develop new drugs, outsource the manufacturing process to CMOs in order to focus their resources on drug discovery and clinical development.
To produce pharmaceutical products, we must have quality systems and equipment that meet manufacturing standards called Good Manufacturing Practice (GMP).
Supporting the development carried out by pharmaceutical companies and raising the possibility for new drugs

The origin of our pharmaceutical development lies in the technologies that we have developed over the years for the synthesis of amino acids and the production of optically active compounds. We have used these technologies to transform Our Business into APIs manufacturing to expand the scope of our contributions to drug development. Currently, we have developed contract manufacturing of active pharmaceutical ingredients (APIs) and pharmaceutical intermediates.
As a CMO, we support drug development from a manufacturing perspective so that new medicines can reach patients quickly and safely, helping to cure diseases and improve patients' quality of life.
Absolute security, guaranteed fast delivery

We collaborates with pharmaceutical companies to bring new medicines to market as quickly as possible for many patients who are waiting for them, as well as to achieve their health. Therefore, we must ensure the absolute safety and high quality of our APIs and intermediates. In order to achieve these goals, we comply with regulations of each country, strictly adhere to GMP-based manufacturing procedures and quality control, and pass global inspections and audits during manufacturing.
In addition, we have established a top-class data integrity system in Japan that is used for quality control of various APIs and intermediates. For these electronic records, we are prepared to ensure the protection and accessibility of accurate and complete records for a specified retention period, and to provide high quality and security at all times.
Meet all needs and maximize value for patients

In order to respond to various diseases, more emphasis has been placed on shortening the time from R&D of drugs with new efficacies to marketing and delivery to patients.
Therefore, the CMOs must have the ability to develop and manufacture in a timely manner in accordance with the pharmaceutical company's development plan. We have many R&D, manufacturing staff and a variety of equipment. In 2023, We have increased our production capacity to meet additional medical needs and new contracts from customers with the addition of the intermediates manufacturing plant. We also have multiple production lines for APIs conform to GMP, including investigational drugs, to meet demand at all scales from investigational to commercial manufacturing. By providing support from R&D to commercial manufacturing, We will continue to respond to customer and medical needs and contribute to the realization of new medical solutions.