- home
- Our Work
Our Work


Job description
Positions available at SEKISUI MEDICAL include roles in R&D, Plant Technology, Sales, and other positions.
-

Research & Development
Active in various businesses such as Diagnositc, Fine Chemical, Drug Development Solutions and Analytical Technology Centre of Drug Development . We also contributewidely in the medical field through drug development, clinical/non-clinical trials, and in terms of pharmaceutical manufacturing.
-

Plant Technology
Active in the Diagnostc and Fine Chemical businesses. For the Diagnostics business, we are responsible for the commercialization of products created by the R&D department. On the pharmaceutical side of the business, we deliver tailor-made raw materials to meet customer needs.
-

Sales
Active in the Diagnostic, Pharmaceutical Sciences (fine chemicals and drug development solutions). For the Diagnostics business sales staff are referred to as DMRs (Diagnostics Medical Representatives) and develop high level expertise in this specialized field. In the Pharmaceutical Science business, sales staff develop deep ties to supply tailored products for major players in the pharmaceutical industry.
-

Others
A variety of other roles are pivotal in the operation of the company including administrative positions (General Affairs, Corporate Planning, Accounting, HR, Legal Affairs, etc.), and QA and facility management positions that ensure our quality provided is consistent.
Research and Development
We work to develop new pipelines for the Diagnostics, Pharmaceuticals & Fine Chemicals, and Drug Development Solustions businesses.

Diagnostics Business
In the clinical diagnostics business, we conduct research and development of clinical diagnostic reagents, along with associated products and analyzers. Our goal is to be pioneers in clinical diagnostic reagents and leaders in the Clinical Chemistry field.
Fine Chemicals
In the Fine Chemicals business, we conduct research and development relating to bulk pharmaceuticals, intermediates, and amino acids necessary for the manufacture of medicines. Using our advanced manufacturing technology for active compounds, we develop tailormade products for clients from pharmaceutical, food, and chemical manufacturers as well as trading companies
Drug Development Solutions Business
In the drug development solutions business, we conduct clinical and non-clinical testing (ADME, metabolite analysis, etc.) and provide test results to our customers in order to support the research and development of drugs and other products. We provide comprehensive support for the entire process of drug development from discovery to reguatory application and post-marketing surveillance.
Analytical Technology Center for Drug Development
The Analytical Technology Research Division is responsible for the development of new analytical technology platforms and products (genetic, immunological, cellular, etc.) necessary for fine chemical and pharmaceutical development.

See interviews with employees working in R&D positions.
-

Research and Development
(Diagnostics)
Joined 2016
-

Research and Development
(Drug Development Solutions)
Joined 2019
-

Research and Development
(Pharmaceuticals & Fine Chemicals)
Joined 2019
-

Research and Development
(Analytical Technology Center for Drug Development)
Joined 2020
Plant Technology
Chemistry, Manufacturing and Control
We work in both Diagnostics and Pharamceutical Sciences businesses.
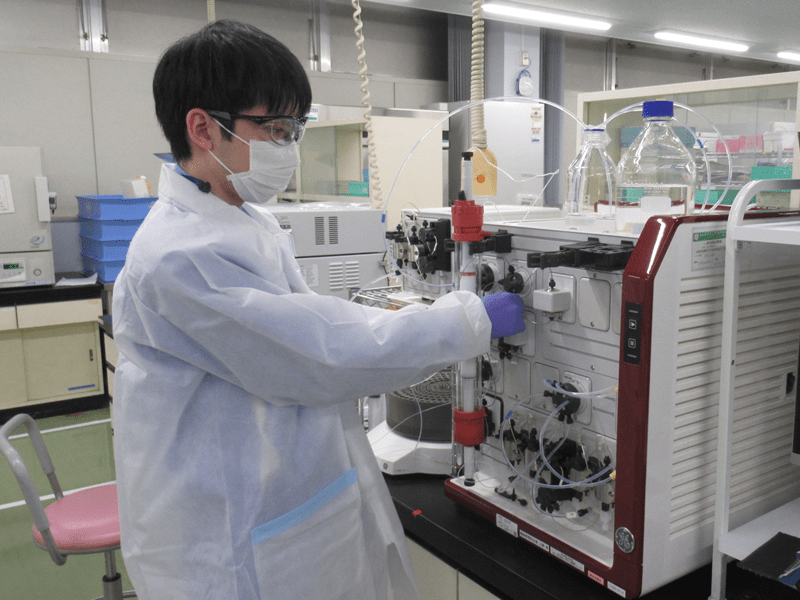
Plant Technology (Diagnostics Business)
Performs duties related to manufacturing, quality control and production technology of diagnostics business products.
《Formulation Department》
Preparation: We dissolve quality-checked raw materials and prepare reagents.
Filling: Reagents are filled into containers suitable for various analytical instruments.
Packaging: Packaging is applied to each product to make it available to customers.
Business improvement: We will work on issues related to each process (improvement of productivity, improvement of manufacturing quality, verification of equipment installation etc.).
《Quality Control Department》
Quality control of raw materials, intermediates, and products. We measure in actual situations to check quality.
《Technology Division》
Product technology: We examine raw materials and improves processes to ensure stable supply.
Transfer technology: We establish a mass production system for products developed in the research laboratory.
Material technology: We establish a production system for self-developed materials such as latex particles and antibodies.
Plant Engineering (Pharmaceutical Business)
In the fine chemical business, we are engaged in manufacturing, production technology, and quality control for contract manufacturing of amino acid. We are also engaged in intermediates and active ingridients required for fine chemical manufacturing.
《Manufacturing Division》
We prepare raw materials in reaction cans and manufactures products through mixing and reactions.
《Production Technology Division》
We conduct laboratory studies of fine chemical manufacturing methods, optimize them for industrialization, and establish industrial manufacturing methods. This is followed by prototype manufacturing at the factory and actual production scale.
《Quality Control Department》
Conducts quality tests on products produced at the factory and on raw materials and water used in manufacturing.


Facilities and equipment management
We manage the machinery and equipment of each research institute and factory.
We maintain safe and secure research and production facilities for product manufacturing.
(Examples of work)
・Facility maintenance: repair and construction management of production facilities
・Capital investment: plant planning and design
・Plant waste management: management of liquid waste and waste from plant production
・Environmental conservation: plant energy reduction
・Utility management: water intake management, boiler management, etc.
Sales Representatives
We are active in the areas of diagnostics, fine chemicals and drug development solutions.

Sales Representative (Diagnostic Business)
The abbreviated name DMR (Diagnostic Medical Representatives) refers to sales representatives for diagnostic reagents. We have two main roles. The first is to provide information on products related to clinical laboratories (diagnostic reagents, testing tools, and automated analyzers) to physicians and clinical laboratory technicians. In addition, we also coordinate customer support with the relevant departments, such as academic and technical support for products, demonstrations, and instrument maintenance, which are works of the Customer Support Center. The second is to bring back to the company the information on the clinical site where the product was actually used for the patient. DMRs are the bridge between customers and the company, and they are indispensable in providing products that meet the needs of clinical practice.
Sales staff (Pharmaceutical Business)
This is a sales position in the fine chemical business and drug development solutions business. In the fine chemical business, our customers are domestic pharmaceutical manufacturers, food and chemical manufacturers, and trading companies. Sales representatives connect customers with our R&D and plant engineering departments, and provide contract services for fine chemicals substances, intermediates, and amino acids required for pharmaceutical manufacturing. In the drug development solutions business, we are commissioned by domestic pharmaceutical manufacturers, venture companies, academia, and other customers to conduct tests in drug development (data required for drug development and approval, such as pharmacokinetics and safety) and connect them to our R&D departments.
Other with administrative and support
We are active in the areas of diagnostic business and fine chemical business.

Business Administration
We support corportate, business, and employees in specialized areas such as human resources, general affairs, accounting, legal affairs, IT, plant management, and sales management. They are indispensable for the smooth operation of each department.
Quality Assurance
We carry out quality assurance related to products, and manage compliance with laws, regulations and GMP (requirements for manufacturing pharmaceutical products set by the government). Qualified pharmacists are also active.









