CHC (Child Health Care) Initiatives
Through clinical tests, Sekisui Medical is working to support the healthy growth of the next generation from pregnancy.

1. Establishment of testing methods for diseases for which a new treatment method has been found
Contributing to the diagnosis of rare diseases through the development of test kits and the consignment of tests using advanced technology
2. Provision of testing technology to medical institutions in Japan and overseas
In order to have as many people as possible undergo the necessary examinations, we are actively expanding our activities both in Japan and overseas.
3. Support for dissemination activities such as diagnosis and treatment of rare diseases
Comprehensive alliance agreement with the National Center for Child Health and Development and collaboration with academic institutions in Japan and overseas
Efforts for expanded newborn screening
Since December 2020, the SMCL Center has been entrusted with the measurement of LSD (Lysosomal Storage Disease) -related enzyme activities in the Dried blood spot (DBS), TREC (T cell receptor excision circles) and KREC (kappa-deleting recombination intersection circles) in the DBS, and SMN1 (Survival of Motor Neuron 1) in the DBS.
Since new drugs have been developed in recent years, it is possible to perform an examination using DBS in the neonatal period as an expanded newborn screening, leading to early detection and early treatment.
- 1Measurement of LSD-related enzyme activity
Lysosomal storage disease (LSD) is a collective term for diseases that occur when a large amount of undegraded material accumulates in the body due to genetic defects in lysosomal degrading enzymes.
Because each disorder has a different enzyme deficiency, measuring the activity of each enzyme in a blood sample enables doctors to screen for disease.
The SMCL Center will contract screening tests for five diseases of LSD: Fabry's disease, Pompe's disease, Gaucher's disease, mucopolysaccharidosis type I, and mucopolysaccharidosis type II. - 2Measurement of TREC/KREC
TREC is circular DNA in which the part encoding the δ chain of the T cell receptor was cut off during the differentiation of T cells, and KREC is circular DNA in which the part encoding the constant part of the κ chain was cut off during the differentiation of B cells.
By measuring TREC and KREC, it has been reported that screening in the neonatal stage of primary immunodeficiencies such as severe combined immunodeficiency (SCID), typical X-linked agammaglobulinemia (XLA) of antibody production deficiency, and ataxia-telangiectasia (AT) associated with immunodeficiency is useful. - 3Measurement of SMN1
The SMN1 gene produces the SMN protein needed to maintain motor neuron function in the spinal cord.
Most cases of spinal muscular atrophy (SMA) are known to be caused by homozygous deletion of the SMN1 gene, and neonatal screening of SMA has been reported to be useful by measuring the SMN1 gene.
Overview of Contract testing
| Contract items | Abbreviation/ Contract code | Method | Sample |
|---|---|---|---|
| Measurement of LSD-related enzymatic actibity in dried blood spot *1 ①α-galactosidase A (GLA) ②α-glucosidase (GAA) ③β-glucocerebrosidase (ABG) ④α-L-iduronidase (IDUA) ⑤iduronate-2-sulfatase (IDS) |
①Fabry : SMD001 ②Pompe : SMD002 ③Gauche : SMD003 ④MPSⅠ : SMD004 ⑤MPSⅡ : SMD005 |
LC-MS/MS | Dried blood spot (DBS) |
| Measurement of TERC/KREC in dried blood spot | TREC : SMD008 KREC : SMD009 |
real-time PCR | Dried blood spot (DBS) |
| Measurement of SMN1 in dried blood spot | SMN1 : SMD010 | real-time PCR | Dried blood spot (DBS) |
- *1Please contact us separately about the combination of items.
- *All inspections are not covered by insurance (for research).
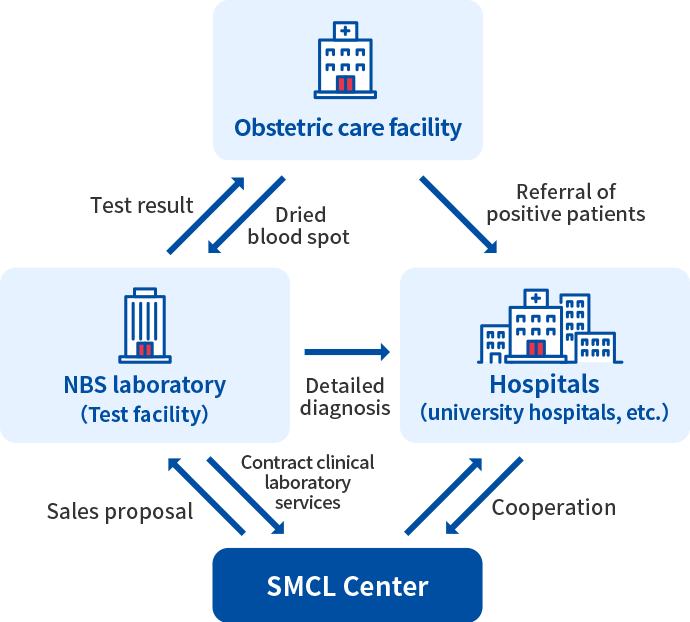
System of expanded newborn screening
It is carried out in each region under the inter-facility cooperation system with major hospitals such as obstetrics medical institutions, NBS laboratory for examinations, and university hospitals for detailed examinations, definitive diagnosis and treatment.
The SMCL Center will cooperate with each facility and doctor to provide contract and sales reagents for expanded newborn screening.
The SMCL Center also sales reagents for newborn mass screening and for expanded newborn screening.
Please check NeoSMAAT, NeoSMAAT T/K/S for details.
"NeoSMAAT" and "NeoSMAAT T/K/S" are registered trademarks of Sekisui Medical Co., Ltd. in Japan.