Clinical Pharmacokinetic Study
We conduct Clinical Pharmacokinetic Study using 14C-labeled compounds at domestic clinical sites.
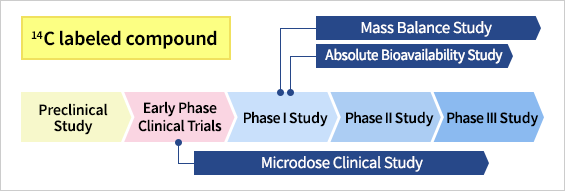
Microdose (MD) clinical study
As a member of the project "Innovative Strategies for Drug Development and Commercialization using Microdosing Clinical Studies" of New Energy Industrial Technology Development Organization (NEDO), we have carried out the first MD clinical study with 14C-labeled compound in Japan. Knowledge we gained through this project allowed us to expand our services to include microdose clinical study using both radiolabeled and unlabeled compounds.
Mass balance study
Mass balance study will be carried out using a formulation containing a trace amount of 14C-lableld GMP active pharmaceutical ingredients as well as a clinical amount of unlabeled counterpart. Both radiolabeled and unlabeled compounds can be produced in our laboratory under GMP compliance. Accelerator Mass Spectrometry (AMS) is used to elucidate mass balance and metabolite profile. In addition, unchanged drug concentration in plasma will be determined by using LC-MS/MS.
Bioavailability study
Absolute bioavailability will be evaluated by combining the ordinal administration of unlabeled compounds through clinical routes with intravenous administration of a trace amount 14C-labeled counterpart. Injections are prepared in an isolator which is installed in our laboratory and then transported to clinical site. To assure the quality of sterile products, various inspections are carried out before shipping