Nanopia PSA
We created this page for medical staff.
- *Read carefully the package insert before use.
Performance Data
Within-run Reproducibility
| n=10 | Measured Value (ng/mL) | ||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
| Mean | 1.05 | 1.77 | 2.56 | 4.52 | 13.81 |
| S.D. | 0.05 | 0.05 | 0.11 | 0.10 | 0.13 |
| CV(%) | 5.0 | 2.7 | 4.2 | 2.3 | 0.9 |
| Min. | 1.0 | 1.7 | 2.4 | 4.3 | 13.5 |
| Max. | 1.1 | 1.8 | 2.7 | 4.7 | 13.9 |
CV is approximately 5% at 1 ng/mL and approximately 3% at around 2 ng/mL, showing good precision.
Reactivities of free and bound PSA
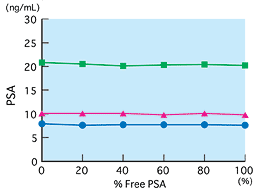
Using free PSA and bound PSA of the same concentration, samples prepared by changing the mixing ratio of free and bound PSA were measured. According to the results, results did not change in the measurement region, from 0 to 100 percent of free PSA. This shows that free and bound forms are measured equally (equimolar response).
Detection limit and precision
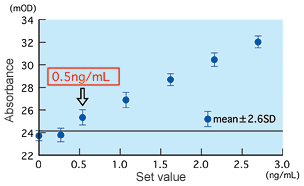
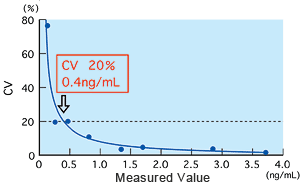
The detection limit was found to be 0.5 ng/mL using the method of Mean ±2.6SD at zero concentration, and 0.4 ng/mL using an effective sensitivity 20 percent of CV for between-run precision. Both results show that the detection limit is approximately 0.5 ng/mL.
Linearity
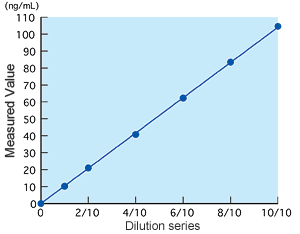
Linearity was observed at a concentration of up to 100 ng/mL.
Correlation
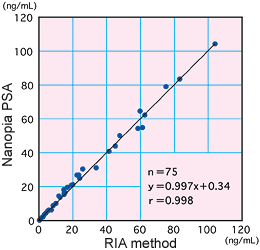
There was high correlation between our method and the RIA method which is accepted as the standard method.
"Nanopia" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.