Nanopia TDM Theophylline
We created this page for medical staff.
- *Read carefully the package insert before use.
Features

- Liquid reagent, ready to use
- Applicable to various automated analyzers
- *This package insert follows the Pharmaceuticals, Medical devices and Other Therapeutic Products Act of Japan.
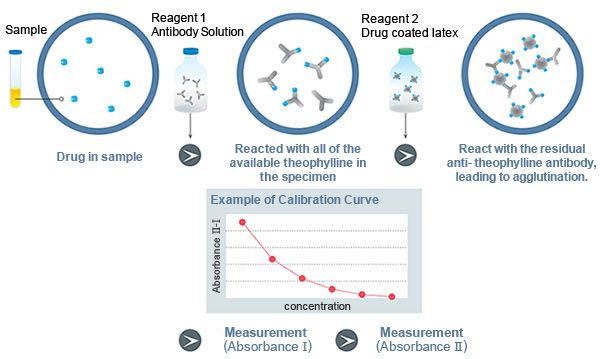
Assay Principle
Latex Immunoturbidimetric Assay
When a certain amount of anti-theophylline antibody is added to a specimen, the anti- theophylline antibody binds to theophylline until it has reacted with all of the available theophylline in the specimen. Next, theophylline -coated latex are added, which react with the residual anti- theophylline antibody, leading to agglutination. Since the extent of agglutination depends on the concentration of theophylline in the specimen, the theophylline concentration can be determined by measuring the absorbance as an indicator of agglutination.
Package
| Product Name | Package | Storage | |
|---|---|---|---|
| Nanopia TDM Theophylline | THE Antibody Solution 1 | 1×36mL | 2-8℃ |
| THE Latex Reagent 2 | 1×6mL | ||
- *Other package sizes are available. Contact SEKISUI MEDICAL CO., LTD. for details.
Sold Separately*
| Product Name | Package | Storage |
|---|---|---|
| TDM Calibrator for Nanopia ** | 1×6conc.×2mL | 2-8℃ |
| TDM Sample Dilution Solution for Nanopia ** | 1×10mL |
- **Not in vitro diagnostic products in Japan.
"Nanopia" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.