Nanopia TDM Phenobarbital
We created this page for medical staff.
- *Read carefully the package insert before use.
Features

- Liquid reagent, ready to use
- Applicable to various automated analyzers
- *This package insert follows the Pharmaceuticals, Medical devices and Other Therapeutic Products Act of Japan.
Assay Principle
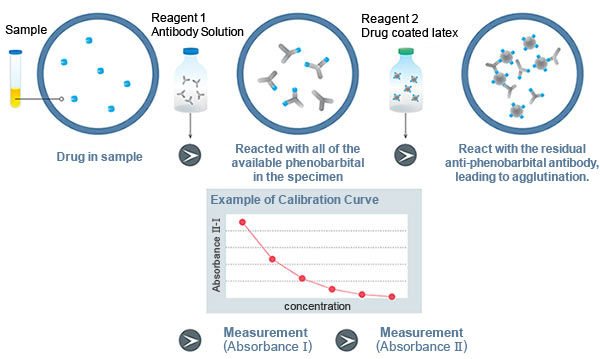
Latex Immunoturbidimetric Assay
When a certain amount of anti- phenobarbital antibody is added to a specimen, the anti- phenobarbital antibody binds to phenobarbital until it has reacted with all of the available phenobarbital in the specimen. Next, phenobarbital-coated latex are added, which react with the residual anti- phenobarbital antibody, leading to agglutination. Since the extent of agglutination depends on the concentration of phenobarbital in the specimen, the phenobarbital concentration can be determined by measuring the absorbance as an indicator of agglutination.
Package
| Product Name | Package | Storage | |
|---|---|---|---|
| Nanopia TDM Phenobarbital | PB Antibody Solution 1 | 1×36mL | 2-8℃ |
| PHT Latex Reagent 2 | 1×10mL | ||
- *Other package sizes are available. Contact SEKISUI MEDICAL CO., LTD. for details.
Sold Separately*
| Product Name | Package | Storage |
|---|---|---|
| TDM Calibrator for Nanopia ** | 1×6conc.×2mL | 2-8℃ |
| TDM Sample Dilution Solution for Nanopia ** | 1×10mL |
- **Not in vitro diagnostic products in Japan.
"Nanopia" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.