Production Division
Equipments of manufactuirng API and Intermediates.
New plant for producing pharmaceutical intermediates
Our plant for manufacturing intermediates with two independent production lines, designed for 1 equipment /1 room, was completed in 2023. We will achieve a 25% increase in production capacity, and will strongly promote the expansion of development pipeline and the expansion of new contracts.

| Item | Main equipment | Material | Size | Number of equipment |
|---|---|---|---|---|
| General Area | Reaction tank | Conductive GL,SUS | 6,000~8,000L | 9 |
| Centrifuge | Conductive TFL | 48B | 4 | |
| Conical dryer | Conductive GL | 3,000L | 2 | |
| Manufactured product | Pharmaceutical intermediates | |||
| Building size | Four-storey steel frame , Floor area 2,150m2 | |||
Pharmaceutical Intermediate Manufacturing Equipment (Multi-Purpose Manufacturing Equipment)
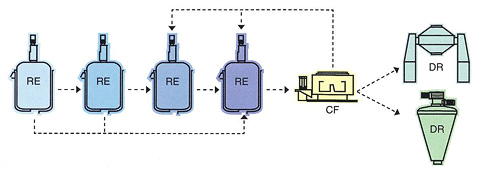

API Manufacturing Equipment (Cleanroom: ISO 8)
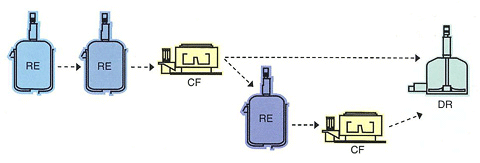

Overview of the Equipment
| Intermediate/crude crystal | API(Cleanroom: ISO 8) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Line 1 | Line 2 | ||||||||
| Jacketed mixing tank (reaction tank) | GL | 3,000 - 4,000L | 2 | GL | 1,500L | 1 | GL | 3,000L | 1 |
| 5,000L | 2 | 7,000L | 1 | 5,000L | 2 | ||||
| 8,000 - 10,000L | 3 | 8,000L | 1 | ||||||
| SUS304 | 3,000 - 4,000L | 3 | SUS304 | 6,000L | 1 | ||||
| SUS316L | 5,000 - 8,000L | 2 | SUS316L | 6,000L | 1 | ||||
| Centrifuge | TFL | 48B | 2 | TFL | 36B | 2 | TFL | 36B | 2 |
| SUS316L | 36B | 1 | |||||||
| Dryer | Conical-type | Conical-type | Pan dryer | ||||||
| NSGL | 3,000L | 1 | NSGL | 3,000L | 1 | SUS316L | 2,100L | 1 | |
| Biaxial stirring dryer | |||||||||
| SUS304 | 3,000L | 1 | |||||||
| Other | Oxygen concentration meter Water for manufacturing purposes, only for the manufacturing buildings (deionized water & UF water) |
||||||||
Investigational New Drug API and Pharmaceutical Intermediate Manufacturing Facilities
The Iwate Plant has several API production lines in operation, and is is equipped with two production lines that conform to the GMP for API and investigational new drugs.
Line 1 is a production line made up of equipment that mainly consists of GL tanks or varying sizes between 100 and 500 liters. It is comprised of manufacturing equipment that is based upon the assumption that it will produce new investigational drugs on a scale of between 10 and 50 kilograms, and handle the process from the reaction to crystallization, separation, and dessication, as well as a product chamber where ISO 8 dust-proofing measures are in force.
Line 2 is a production line composed of equipment with a maximum of between 1 and 1.5 tons. It manufactures APIs on a scale of between 50 and 100 kilograms in an integrated process from the reaction to crystallization, separation, and desiccation. It has an ISO 8 product chamber.
List of Line 1 Equipment
| Type | Material | Size | No. | |
|---|---|---|---|---|
| Reaction tank/Crystallization tank | SUS304 SUS316L GL, NSGL |
500L 500L 100 - 500L |
1 1 7 |
|
| High-pressure reaction tank | SUS304 SUS304 |
800L 100 - 500L |
1 2 |
Press 780Kpa |
| High-vacuum distillation towers | Glass | 200L | 1 | |
| Centrifuge | TFL TFL |
30B 24B |
1 1 |
|
| Dryer | NSGL TFL |
300L 65L |
1 1 |
Conical-type Vibration dryer |

List of Line 2 Equipment
| Type | Material | Size | No. | |
|---|---|---|---|---|
| Reaction tank/Crystallization tank | GL GL GL SUS316L |
500L 1,000L 1,500L 800L |
1 1 1 1 |
|
| Centrifuge | SUS304 PFA/SS400 |
30B 30B |
1 1 |
|
| Carbon filter | HC22/SS400 | 0.5m2 | 1 | |
| Dryer | PFA/SUS304 | 240L | 1 | Vibration dryer |

Cleanroom Annex
| Item | Main equipment | Material | Size | Number of equipment |
|---|---|---|---|---|
| General area | Reaction tank | Conductive GL | 3,000L | 1 |
| Cleanroom | Crystallization tank | Conductive GL | 3,000L | 1 |
| Centrifuge | Conductive TFL | 36B | 1 | |
| Conical dryer | Conductive GL | 2,000L | 1 | |
| Manufactured product | APIs, investigational new drugs | |||
| Building size | 3-storey steel frame building with a total floor space of 1,230 m2 | |||


