Quality Assurance Division
GMP management system in Iwate Plant
Extensive Authority Inspection Experience and System Management
Based on our extensive experience, we have established a strong GMP management system, have continuously maintained and updated the required GMP system for APIs and intermediates on a daily basis. By complying with the laws and regulations of various countries including the Japanese authorities, the U.S. Food and Drug Administration and the European Medicines Agency, Sekisui Medical continues to expand our contract manufacturing business for high-purity APIs on a global scale.
Robust Quality Management System
To ensure data integrity, Sekisui Medical has an audit trail system compliant with 21 CFR Part 11 and various analytical instruments that have undergone appropriate computer system validation, which are utilized for quality control of various APIs and intermediates.
These electronic records ensure the protection and accessibility of accurate and complete records for a specified retention period.
With other various analytical instruments, Sekisui Medical has a great reputation with customers and authorities for implementing appropriate quality control through Laboratory Information Management System (LIMS).
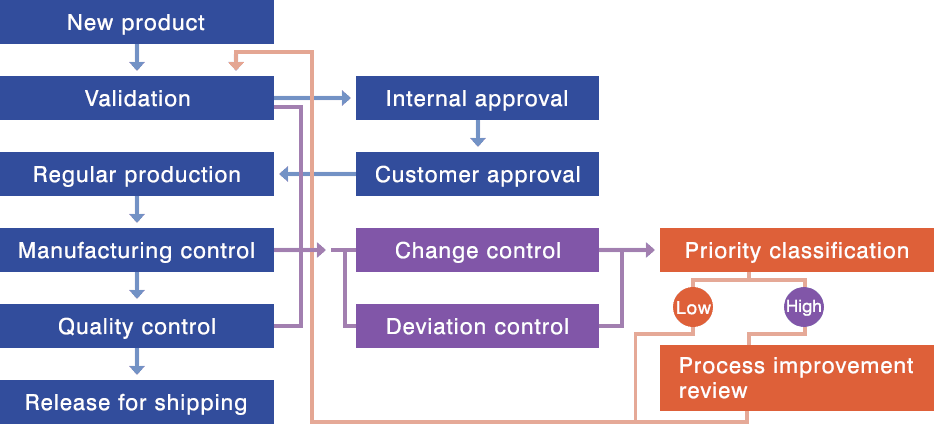
Our Quality Control System
We have in place the GMP system required for the manufacture of investigational new drugs and pharmaceuticals, and carry out GMP-compliant quality control for pharmaceutical intermediates as well. We register our DMFs (drug master files) with the FDA.
Authority Inspection History
| Timeline | Type of inspection |
|---|---|
| 1984 | Underwent first FDA inspection. |
| 2008 | Underwent EDQM inspection. |
| 2011 | Underwent our 9th FDA inspection (in October). |
| 2016 | Underwent 10th FDA inspection (in June). |
| 2017 | Underwent 11th FDA inspection (in July). |
| 2019 | Underwent 12th FDA inspection (in March). |