Nanopia IL-2R
We created this page for medical staff.
- *Read carefully the package insert before use.
Assay Procedure
Reagent Preparation
- Reagent 1: IL-2R Buffer Solution 1 is ready to use.
- Reagent 2: IL-2R Latex Reagent 2 is ready to use.
Before using this product, gently invert the IL-2R Latex Reagent 2 bottle to mix it thoroughly and check that there are no bubbles.
Assay Procedure
This product is compatible with various automated analyzers. An example of the assay procedure is indicated below.
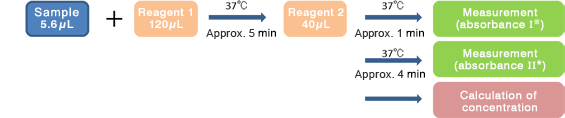
- *AbsorbanceⅠandⅡ: Difference between absorbance at 570nm and 800nm
Calibration material: IL-2R Calibrator for Nanopia (in-house indicated values)
Reagent blank: Purified water or saline
Assessment of Assay Results
- 1Reference Interval
Each institution should determine its own reference interval, because this may be influenced by various factors that can vary among institutions. A reference range of 122-496 U/mL has been reported .
References:Medical Practice Editing Committee: Guide for Laboratory Tests, 2015 revision 1014 (2015). - 2Precautions for Assessment
There may be reactions with non-target substances or interfering reactions. If assay results appear unreliable, repeat the measurement (if necessary, after dilution) or try another analytical method.
"Nanopia" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.