Arbekacin Kit
Nanopia TDM Arbekacin
We created this page for medical staff.
- *Read carefully the package insert before use.
Performance Data
On Hitachi 7180(except for the correlation)
Within-run Reproducibility
| Measured Value (ng/mL) | |||
|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | |
| n | 10 | 10 | 10 |
| Mean | 4.0 | 12.5 | 25.7 |
| SD | 0.03 | 0.12 | 0.48 |
| CV(%) | 0.8 | 1.0 | 1.9 |
| Max. | 4 | 12.3 | 25.3 |
| Min. | 3.9 | 12.7 | 26.5 |
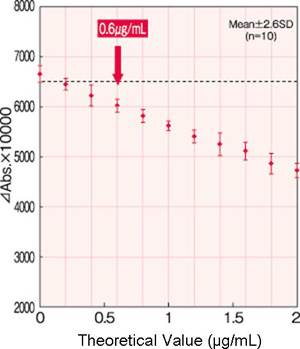
Detection Limit
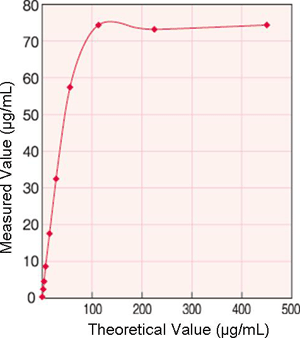
Prozone
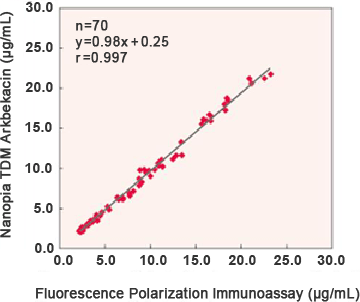
Correlation
Interference
| Spiked Concentration | Measured Value (ng/mL) | ||
|---|---|---|---|
| Base Sample | Spiked Sample | ||
| Unconjugated Bilirubin | 19.4mg/dL | 4.9 | 4.9 |
| Conjugated Bilirubin | 20.3mg/dL | 4.8 | 4.9 |
| Hemoglobin | 491mg/dL | 4.9 | 4.9 |
| Rheumatoid factor | 500IU/mL | 5.2 | 5.2 |
| Chyle | 1410 FTU | 4.9 | 4.9 |
| Intralipos | 1.0% | 4.8 | 4.9 |
Measurement Range (Hitachi 7180)
Detection limit: 0.6µg/mL
Upper limit: 30.0µg/mL
"Nanopia" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.