Cholestest N HDL
We created this page for medical staff.
- *Read carefully the package insert before use.
Reference Data (In-house data of SEKISUI MEDICAL)
On Hitachi 7180
Within-run Reproducibility
| Sample 1 | Sample 2 | Sample 3 | |
|---|---|---|---|
| n | 20 | 20 | 20 |
| Mean | 38.72 | 90.78 | 53.77 |
| S.D. | 0.38 | 0.54 | 0.47 |
| C.V. | 0.98 | 0.59 | 0.87 |
| Max. | 39.6 | 91.9 | 54.5 |
| Min. | 38.1 | 89.8 | 53.0 |
| Range | 1.5 | 2.1 | 1.5 |
Linearity
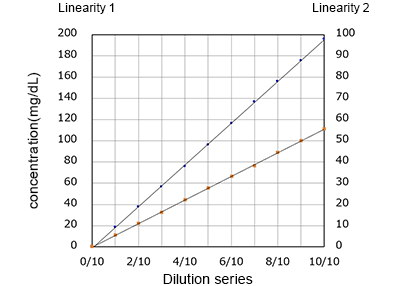
Correlation
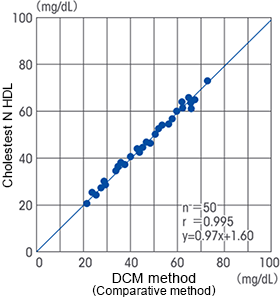
The HDL-cholesterol direct assay reagent "Cholestest N HDL" manufactured and marketed by SEKISUI MEDICAL CO., LTD., is designed to comply with the DCM method (dextran sulfate magnesium precipitation separation method).
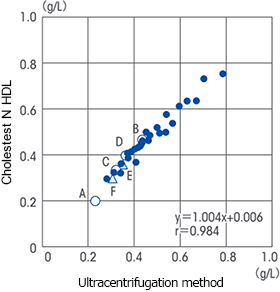
It has been reported that the correlation with the ultracentrifugation method was not observed in serum samples (TG 259 - 1497 mg/dL) of patients with hypertriglyceridemia.
<References * 1> Yoshikazu Ono, et al., Clinical Science 2002: 31: 44 -50.
Interference
Unconjugated Bilirubin
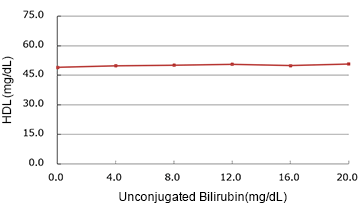
| Spiked Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0.0 | 49.00 | 100.0% |
| 4.0 | 49.80 | 101.6% |
| 8.0 | 50.10 | 102.2% |
| 12.0 | 50.60 | 103.3% |
| 16.0 | 49.90 | 101.8% |
| 20.0 | 50.75 | 103.6% |
Conjugated Bilirubin
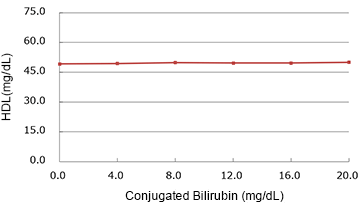
| Spiked Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0.0 | 49.30 | 100.0% |
| 4.0 | 49.40 | 100.2% |
| 8.0 | 49.90 | 101.2% |
| 12.0 | 49.70 | 100.8% |
| 16.0 | 49.75 | 100.9% |
| 20.0 | 50.00 | 101.4% |
Hemoglobin
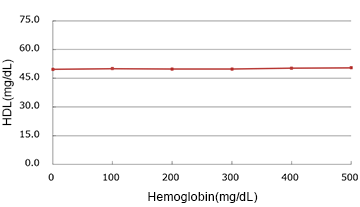
| Spiked Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0 | 49.65 | 100.0% |
| 100 | 50.00 | 100.7% |
| 200 | 49.85 | 100.4% |
| 300 | 49.90 | 100.5% |
| 400 | 50.25 | 101.2% |
| 500 | 50.50 | 101.7% |
Ascorbic Acid
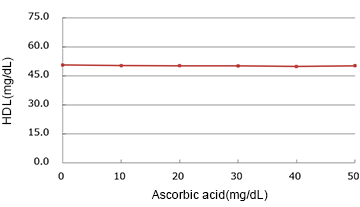
| Spiked Concentration (mg/dL) |
Measured Value | % |
|---|---|---|
| 0 | 50.55 | 100.0% |
| 10 | 50.35 | 99.6% |
| 20 | 50.20 | 99.3% |
| 30 | 50.15 | 99.2% |
| 40 | 49.90 | 98.7% |
| 50 | 50.20 | 99.3% |
Chyle
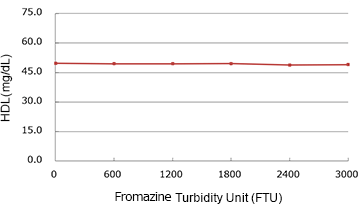
| Spiked Concentration(FTU) | Measured Value | % |
|---|---|---|
| 0 | 49.75 | 100.0% |
| 600 | 49.45 | 99.4% |
| 1200 | 49.40 | 99.3% |
| 1800 | 49.55 | 99.6% |
| 2400 | 48.90 | 98.3% |
| 3000 | 48.95 | 98.4% |
Measurement Range (Hitachi 7170)
2-150mg/dL
"Cholestest" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.