Albumin kit
NORUDIA U-ALB
We created this page for medical staff.
- *Read carefully the package insert before use.
Features
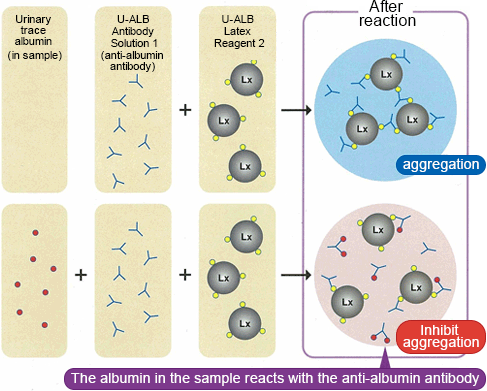
- No prozone effect with the use of the latex immunoturbidimetric method
- Designed for the sensitive measurement of trace urinary albumin
- *This package insert follows the Pharmaceuticals, Medical devices and Other Therapeutic Products Act of Japan.
Assay Principle
Latex Immunoturbidimetric Assay (Competitive Method)
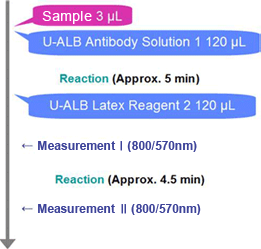
Procedure
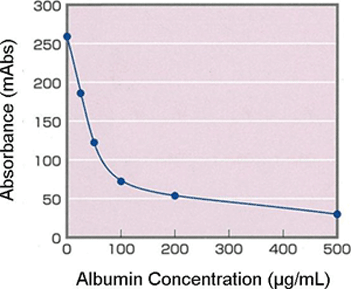
Example of Calibration Curve
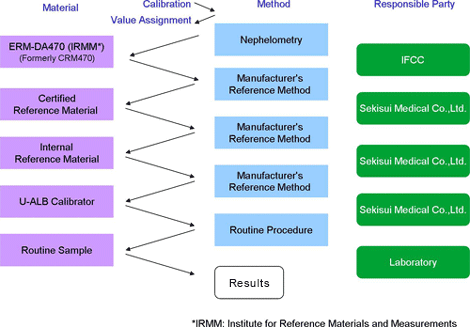
Traceability
Diabetic Neuropathy: Classification and Treatment*1
| Stage | Urinary Protein (Albumin) |
GFR (Kidney Function) |
Treatment |
|---|---|---|---|
| Stage 1 (Pre-nephropathy) |
Normal | Normal Occasionally high |
Glycemic control |
| Stage 2 (Early stage nephropathy) |
Trace albumin | Normal Occasionally high |
Strict glycemic control Antihypertensive therapy |
| Stage 3 A (Early stage chronic nephropathy) |
Persistent proteinuria | Mostly normal | Strict glycemic control Antihypertensive therapy, Protein restriction |
| Stage 3 B (Late stage chronic nephropathy) |
Persistent proteinuria | Low | Strict antihypertensive therapy Protein restriction |
| Stage 4 (Renal failure) |
Persistent proteinuria | Significantly low | Strict antihypertensive therapy Low protein diet, dialysis |
| Stage 5 (Dialysis) |
Receiving dialysis treatment | Organ transplant | |
Reference:
- *1Joint Committee on Diabetic Nephropathy: The Japanese Journal of Nephrology 2002; 44 (1)
Reference Interva*2
| 24 hr Urine Test (mg/24 hr) |
Random Urine Collection (mg/gCr) |
|
|---|---|---|
| Normal | <30 | <30 |
| Trace Urinary Albumin | 30-299 | 30-299 |
| Clinical Proteinuria | ≧300 | ≧300 |
Reference:
- *2The Japan Diabetes Society Guideline on Diabetes Treatment Based on Scientific Evidence [2nd Edition Revised (2007)] p77-92
| Product Name | Package | Storage | |
|---|---|---|---|
| NORUDIA U-ALB | U-ALB Antibody Solution 1 | 1×10mL | 2-10℃ |
| U-ALB Latex Reagent 2 | 1×10mL | 2-10℃ | |
| U-ALB Calibrator A for NORUDIA U-ALB* | 1 × 5conc. × 2mL | 2-10℃ | |
| U-ALB Calibrator B for NORUDIA U-ALB* | 1 × 6conc. × 2mL | 2-10℃ | |
| U-ALB Control for NORUDIA U-ALB* | 3 × 2conc. × 2mL | 2-10℃ | |
- *Not in vitro diagnostic products in Japan.
"NORUDIA" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.