Lupus Anticoagulant (LA) kit
Coagpia LA
We created this page for medical staff.
- *Read carefully the package insert before use.
Features
- Samples taken during warfarin treatment can also be accurately measured.
The measurement is less affected even with intake of warfarin by pre-diluting with a proprietary diluent. - There are two specialized controls. LA positive/LA negative (proprietary diluent)* are available.
*sold separately - Coagpia LA can be measured with the blood coagulation analyzer CP 3000 of the Coapresta series.
Assay Principle
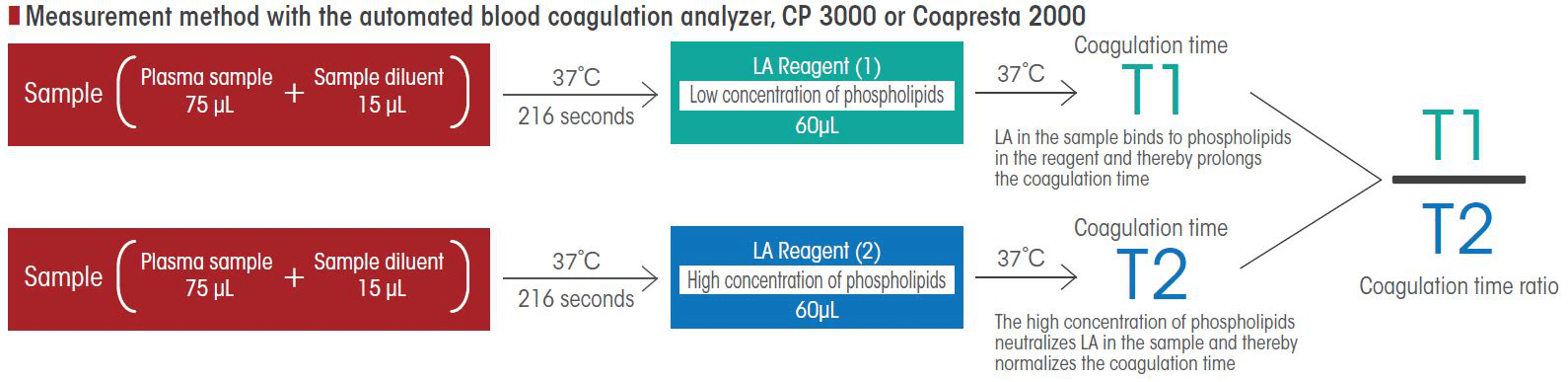
- LA Reagent (1): Phospholipid (low concentration) ⇒ In the presence of LA, the phospholipid is neutralized and tends to be prolonged.
- LA Reagent (2): Phospholipid (high concentration) ⇒ Sufficient amount of phospholipid to neutralize LA, and coagulation time is normalized.
Package
| Product Name | Package | Storage | Stability | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unopened | Opened | ||||||||||||||||||
| Coagpia LA | LA Reagent 1 Phospholipids (low concentration), Russell snake venom |
1×2mL (lyophilized) |
2-10℃ | 23 months | -20℃: 1 Month 2-8℃ :7 Days |
||||||||||||||
| LA Reagent 2 Phospholipids (high concentration), Russell snake venom |
1×2mL (lyophilized) |
||||||||||||||||||
| Sample diluent | 1×1mL (lyophilized) |
||||||||||||||||||
| LA Control (*sold separately) | 5× 2 types× 0.5mL | ||||||||||||||||||
- *The above products are registered as IVD in Japan.
Specifications
| Measuring Method | dRVVT (blood clotting time) | |||
|---|---|---|---|---|
| Sample type | Citrate Plasma | |||
| Reference Method | T1/T2 | |||
| Positive | ≧1.3 | |||
| Negative | <1.3 | |||
| Remarks | If there is a difference of more than 2 seconds between R1 and R2 in normal plasma, use Normalized ratio. | |||
"コアグピア", "Coagpia" and “Coapresta” are trademarks or registered trademarks of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.