Nanopia P-FDP
We created this page for medical staff.
- *Read carefully the package insert before use.
What's FDP?
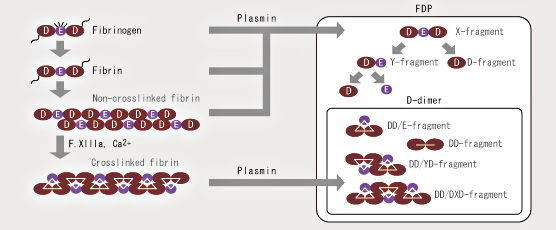
FDP is largely classified into plasmin degradation products of stable fibrin (D-dimer), fibrinogen, and degradation products of unstable fibrin (X, Y, D, and E fractions). It is necessary to capture the various fractions as indicated in the figure when measuring FDP.
Nanopia P-FDP utilizes a monoclonal antibody specific to the D fraction of FDP, which reacts with all fractions of FDP except the E fraction. Thus, the correlation with reagent for measuring FDP in serum used in the conventional method is comparatively high.
Nanopia P-FDP is suitable for use with plasma or serum samples
| FDP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fibrinogen | SF(FM) | High molecular weight FDP | X fraction | Y fraction | DD fraction | E fraction | ||
| Serum FDP | + | + | + | + | + | + | + | + |
| Nanopia P-FDP | - | - | + | + | + | + | + | - |
Nanopia P-FDP utilizes an antibody reactive to the D fraction of FDP and does not react with fibrinogen, thus allowing FDP measurement with both plasma and serum samples.
Problems with serum samples
Antibodies used in conventional FDP measurement react with fibrinogen; therefore, serum samples are used instead of plasma samples. However, serum samples pose the following problems:
- Fibrinogen may remain in sera → High FDP level
- FDP may be contained in blood clots → Low FDP level
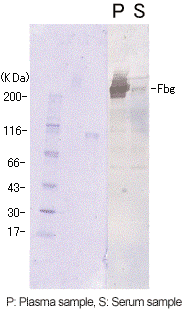
Example of residual fibrinogen
| Reagent | Serum FDP | Nanopia P-FDP | D-dimer | |
|---|---|---|---|---|
| Sample | Serum | Serum | Plasma | Plasma |
| Measured Value (µg/mL) |
56.2 | 5.6 | 7.6 | 2.7 |
A high FDP value was obtained with a conventional reagent for measuring FDP present in serum, but a low FDP value with Nanopia P-FDP. A Western blot analysis was carried out to investigate these divergent results. A band of fibrinogen was also detected within the serum sample. This suggests that with the reagent for measuring FDP present in serum, the measurement of residual fibrinogen caused the high FDP value.
Nanopia P-FDP does not cross-react with fibrinogen, thus allowing measurement using plasma, without being affected by residual fibrinogen in serum.
(Data from Nihon University Itabashi Hospital)
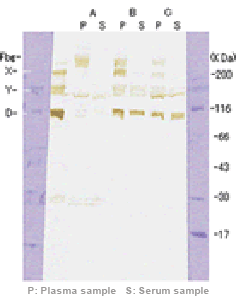
Example of FDP containment
| Sample | Sample A | Sample B | Sample C | |||
|---|---|---|---|---|---|---|
| Plasma | Serum | Plasma | Serum | Plasma | Serum | |
| Measured Value (µg/mL) |
65 | 53 | 79 | 47 | 94 | 59 |
We prepared pseudo-serum by adding Ca2+ and thrombin to plasma. A, B, and C shown here are samples that produced lower values for serum than those for plasma. For all the samples, the bands of fibrinogen disappeared in serum and the bands of X and Y fractions are lighter in plasma than in serum. D fraction bands, however, did not disappear. It is suggested that for these samples, lower levels of FDP were detected in serum than in plasma as some FDP molecules were incorporated into fibrin clots during plasma-serum exchange.
Presence of FDP indicates fibrinolysis in vivo
| Infection | Gram-negative bacterial infection, severe gram-positive bacterial infection, severe viral infection |
|---|---|
| Shock | |
| Malignant tumor | Cancer, Sarcoma, Leukemia |
| Liver disorder | Liver cirrhosis, Fulminant hepatitis |
| Obstetrical disease | Premature separation of placenta, amniotic fluid embolization, retention of dead fetus, hydatidiform mole, pregnancy toxicosis |
| Intravascular hemolysis | |
| Tissue damage | After major surgery (surgery on liver, prostate, pancreas, and adrenal gland, prolonged extracorporeal circulation, extensive trauma, burn) |
| Vascular lesion | Kasabach-Merritt syndrome, cardiac aneurysm, aortic aneurysm, thrombotic thrombocytopenic purpura, collagen disorder, hemolytic disease |
| Others | Organ transplant rejection, administration of a venom drug (defibrase), and thrombolytic therapy |
FDP measurement is one of the tests for DIC diagnosis
In 1988, Ministry of Health, Labor and Welfare proposed the revised diagnostic criteria of DIC (disseminated intravascular coagulation) and in 1992, The Study Group of Blood Clotting Disorders proposed the Matsuda draft proposal. Both bodies adopted the FDP test for the diagnosis of DIC.
| FDP Score | Plasma FDP(Nanopia P-FDP) | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Serum FDP | 0 | 206 | 33 | 0 | 0 | 239 |
| 1 | 6 | 44 | 10 | 0 | 60 | |
| 2 | 4 | 9 | 43 | 4 | 60 | |
| 3 | 0 | 0 | 5 | 29 | 34 | |
| Total | 216 | 86 | 58 | 33 | 393 | |
DIC diagnostic criteria (Matsuda draft proposal)
| Presence of disease causing DIC | ||||
|---|---|---|---|---|
| PLT (/L) | < 100,000 | 100,000 - 150,000 | ≧ 150,000 | |
| FDP | ≧ 10 µg/mL < 20 µg/mL |
Suspected DIC | DIC cannot be denied | |
| ≧ 20 µg/mL < 30 µg/mL |
DIC | Suspected DIC | DIC cannot be denied | |
| ≧ 30 µg/mL | DIC | DIC | Suspected DIC | |
"Nanopia" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.