NORUDIA N HbA1c
We created this page for medical staff.
- *Read carefully the package insert before use.
Performance Data
Within-run Reproducibility
| NORUDIA N HbA1c (NGSP%) |
|||
|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | |
| 1 | 4.7 | 6.3 | 8.8 |
| 2 | 4.7 | 6.4 | 8.9 |
| 3 | 4.8 | 6.4 | 8.9 |
| 4 | 4.7 | 6.4 | 8.8 |
| 5 | 4.8 | 6.4 | 8.9 |
| 6 | 4.7 | 6.4 | 8.9 |
| 7 | 4.7 | 6.4 | 8.9 |
| 8 | 4.8 | 6.4 | 8.9 |
| 9 | 4.7 | 6.4 | 8.8 |
| 10 | 4.8 | 6.4 | 8.9 |
| Mean | 4.74 | 6.39 | 8.87 |
| S.D. | 0.05 | 0.03 | 0.05 |
| C.V.(%) | 1.09 | 0.49 | 0.54 |
| Max. | 4.8 | 6.4 | 8.9 |
| Min. | 4.7 | 6.3 | 8.8 |
| Range | 0.1 | 0.1 | 0.1 |
Between-run Reproducibility
| NORUDIA N HbA1c (NGSP%) |
|||
|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | |
| 1 | 5.0 | 7.0 | 10.3 |
| 2 | 4.9 | 7.0 | 10.3 |
| 3 | 4.9 | 6.9 | 10.2 |
| 4 | 5.0 | 7.1 | 10.4 |
| 5 | 4.9 | 7.0 | 10.3 |
| 6 | 5.0 | 6.9 | 10.3 |
| 7 | 4.9 | 7.0 | 10.2 |
| 8 | 4.9 | 7.0 | 10.2 |
| 9 | 4.9 | 7.0 | 10.3 |
| 10 | 5.0 | 7.0 | 10.4 |
| Mean | 4.94 | 6.99 | 10.29 |
| S.D. | 0.05 | 0.06 | 0.07 |
| C.V.(%) | 1.0 | 0.8 | 0.7 |
| Max. | 5.0 | 7.1 | 10.4 |
| Min. | 4.9 | 6.9 | 10.2 |
| Range | 0.1 | 0.2 | 0.2 |
Measurement of Standard Material*
- *JCCRM 411-3 (JSD Lot5)
| Certified Value and Uncertainty (NGSP%) |
NORUDIA N HbA1c Value (NGSP%) |
|
|---|---|---|
| Level 1 | 5.10±0.13 | 5.08 |
| Level 2 | 5.77±0.14 | 5.74 |
| Level 3 | 7.39±0.19 | 7.38 |
| Level 4 | 9.60±0.23 | 9.62 |
| Level 5 | 11.98±0.28 | 12.12 |
Measurement Range
Hitachi 7170 S
HbA1c(NGSP%) : 3.3-16.6 %
Hemoglobin concentration : 90-310 µmol/L
Interference
| Interfering substances | Spiked Concentration | HbA1c Value (NGSP%) | |
|---|---|---|---|
| Base Sample | Spiked Sample | ||
| Unconjugated Bilirubin | 50mg/dL | 6.6 | 6.5 |
| Conjugated Bilirubin | 50mg/dL | 6.6 | 6.6 |
| Absorbic Acid | 50mg/dL | 6.6 | 6.6 |
| Chyle | 3000 FTU | 6.6 | 6.5 |
Effects of modified hemoglobin and unstable hemoglobin
| Interfering substances | Spiked Concentration | HbA1c Value (NGSP%) | |
|---|---|---|---|
| Base Sample | Spiked Sample | ||
| Acetylated hemoglobin | 50mg/dL | 6.5 | 6.5 |
| Carbamylated hemoglobin | 50mg/dL | 6.5 | 6.5 |
| Acetaldehyde- hemoglobin | 50mg/dL | 6.5 | 6.5 |
| Unstable hemoglobin | 1000mg/dL | 6.5 | 6.5 |
Correlation
- KO500 Method (Hitachi 917)
Hitachi 7170S
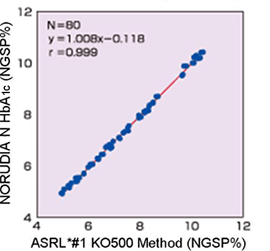
- *Aslan Secondary Reference Laboratory
(Asian Standard Measurement Facilities)
- IFCC Method (Hitachi 917)
Hitachi 7170
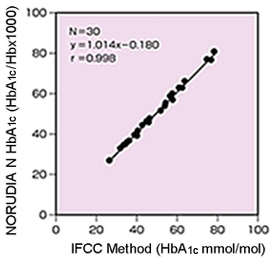
It is easy for NORUDIA N HbA1c to trasition to IFCC method value.
Change in Cell Blank Value
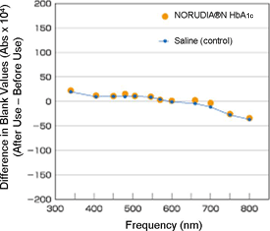
Hitachi 7170S
Blank values before and after 25 assays were measured and compared. Values using NORUDIA N HbA1c was nearly equivalent to that of saline at all frequencies and no latex contamination of the reaction cells were observed.
On-board Stability
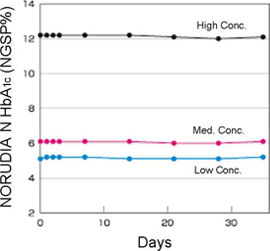
Hitachi 7180
(with HbA1c measurement function option)
- Calibrated on day 0 only
- 2-point calibration (3-point calibration for select devices)
"NORUDIA" is a trademark or registered trademark of SEKISUI MEDICAL CO., LTD. in Japan and/or other countries.
We prepared this page for medical staff (doctors, dentists, pharmacists, clinical laboratory technicians, nurses, etc.) in Japan to ensure the correct use of our products. You should be aware that the information provided is not intended for overseas medical staff and the general public.